Bridging the GxP Gap between Quality and IT
Pharma: Engaging in suppliers is a costly exercise that requires collaboration from both sides: learn how to optimise this relationship for your mutual cost benefits; regulatory GxP compliance and end patient health. We can explain your supplier's technical jargon in plain language; identify regulatory issues; determine underlying quality concerns and provide effectively mitigations to close them. Leverage the knowledge from our specialists who have performed the work of your suppliers across multiple industries and technologies.
Suppliers: We can advise you as to why your customers ask for certain things - and show you how you can meet their needs efficiently, effectively and with low cost. Discover how to be your customer's partner in meeting their regulatory needs. We know what your customers require from over 300 global Computerised System audits.
- We're different because we have a technical and quality background. We operate in the grey area that exits between Quality and IT groups.
- In our previous lives, we have analysed, designed, coded, tested, installed, maintained, migrated and decommissioned complex computerised systems across the globe. We have devised efficient IT Quality Systems to optimise these tasks.
- We became specialists in Computerised System Quality Assurance, taking intimate knowledge of good process and aligning them to regulatory compliance.
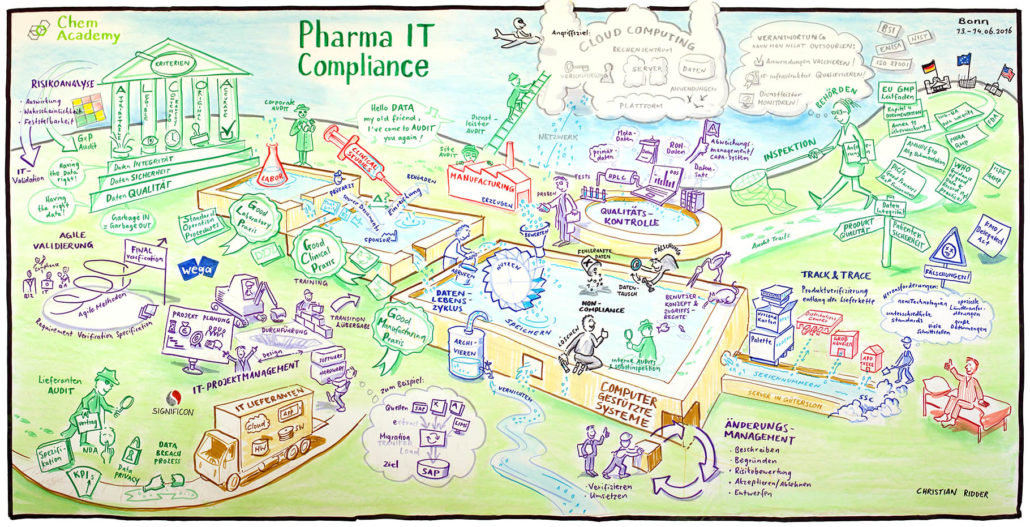
Christian Ridder's graphic, below, displays the areas where we can help.